Reuters reports there are 2.1 Billion people suffering from obesity about the globe and obesity related disease costs exceeded $2 Trillion USD in 2015. McKinsey 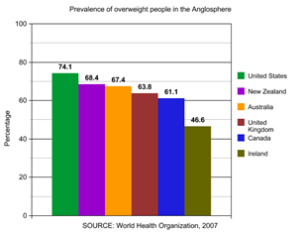 Global Institute forecasts the obesity market to reach $17 trillion USD by 2030 when half of the population is forecast to be obese. These obesity-related diseases include sleep apnea, gastric reflux, hypertension, autoimmune diseases and cancers, vascular disease, heart attacks and strokes, and most importantly type 2 diabetes.
Global Institute forecasts the obesity market to reach $17 trillion USD by 2030 when half of the population is forecast to be obese. These obesity-related diseases include sleep apnea, gastric reflux, hypertension, autoimmune diseases and cancers, vascular disease, heart attacks and strokes, and most importantly type 2 diabetes.
The W.H.O. reports that the U.S. has the highest overweight and obesity proportion in the Anglosphere. Over 2/3 of our population is overweight and 43% is forecast to be obese in a few years. 43% of blacks and Hispanics already are! An individual’s lifetime costs of obesity are estimated at $92,235. 80% of type 2 diabetics are obese as obesity is the leading cause of type 2 diabetes. Diabetes mellitus is the leading cause of renal failure needing dialysis, a frequent cause of blindness, and the most frequent indication for amputations. Obesity doesn’t just shorten your life, but it lessens its quality.
There are 20M diagnosed type 2 diabetics in the U.S. alone and an estimated 9M undiagnosed. Diet and Exercise seldom works for the long haul. Obesity medical device expenditures exceeded $1.4 Billion in 2015 but the annual number of bariatric procedures have plateaued at approximately 220,000 per year because of the complications and lifestyle tradeoffs of the current bariatric surgical alternatives are unpalatable to both patients and doctors. There is an enormous unmet need in developed countries and a large potential in lower income countries due to the cost-effective nature of the device for a minimally invasive treatment of obesity that is safer and highly effective.
 Visceral or “belly” fat not only causes sleep apnea and gastric reflux but secretes the cellular hormones that are responsible for all the complications of obesity – diabetes, hypertension, vascular disease, strokes, heart attacks, autoimmune diseases, and cancers. Patented and proprietary methods and devices have been prototyped and are being tested. We believe our device is distinct from and will be superior to all current or experimental therapies because there is no need to cut into the stomach or bowel, rearrange the alimentary tract, leave behind a foreign body, or be limited by a weight loss plateau. EVL is “safer by design.”
Visceral or “belly” fat not only causes sleep apnea and gastric reflux but secretes the cellular hormones that are responsible for all the complications of obesity – diabetes, hypertension, vascular disease, strokes, heart attacks, autoimmune diseases, and cancers. Patented and proprietary methods and devices have been prototyped and are being tested. We believe our device is distinct from and will be superior to all current or experimental therapies because there is no need to cut into the stomach or bowel, rearrange the alimentary tract, leave behind a foreign body, or be limited by a weight loss plateau. EVL is “safer by design.”
It is anticipated the patient will get off the table in a healthier state and lose 40-90 pounds of body fat following a short ambulatory procedure in which 6-9 pounds of visceral fat is removed. And if necessary, the proposed procedure may be repeated until ideal weight is obtained. We have been granted three (3) U.S. patents (8,348,929B2, 8,465,471B2, and 8,574,223) on both method and the device and numerous other patents are pending. The Company has a qualified tier 2 Offering for $5,000,000 and plans an application for listing on OTC-QB in Q1-Q2 2017. The use of proceeds will include completion of prototype development and obtaining FDA and preliminary European Union approvals.
